Share This Article
The Food and Drug Administration (FDA) confirmed on Wednesday that 37 babies from 17 states are suspected or confirmed to have infant botulism and confirmed exposure to an infant formula.
FDA confirmed in its recent update that the formula was from ByHeart Whole Nutrition.
Cases from 17 states
Laboratory confirmation is still in progress for certain cases. Among the 36 cases where the date of illness onset is known, the illnesses began between August 9 and November 19, 2025.
Fortunately, there have been no reported deaths so far.
For the 35 infants with available age and sex data, their ages range from 16 to 264 days, with 15 (43 percent) being female.
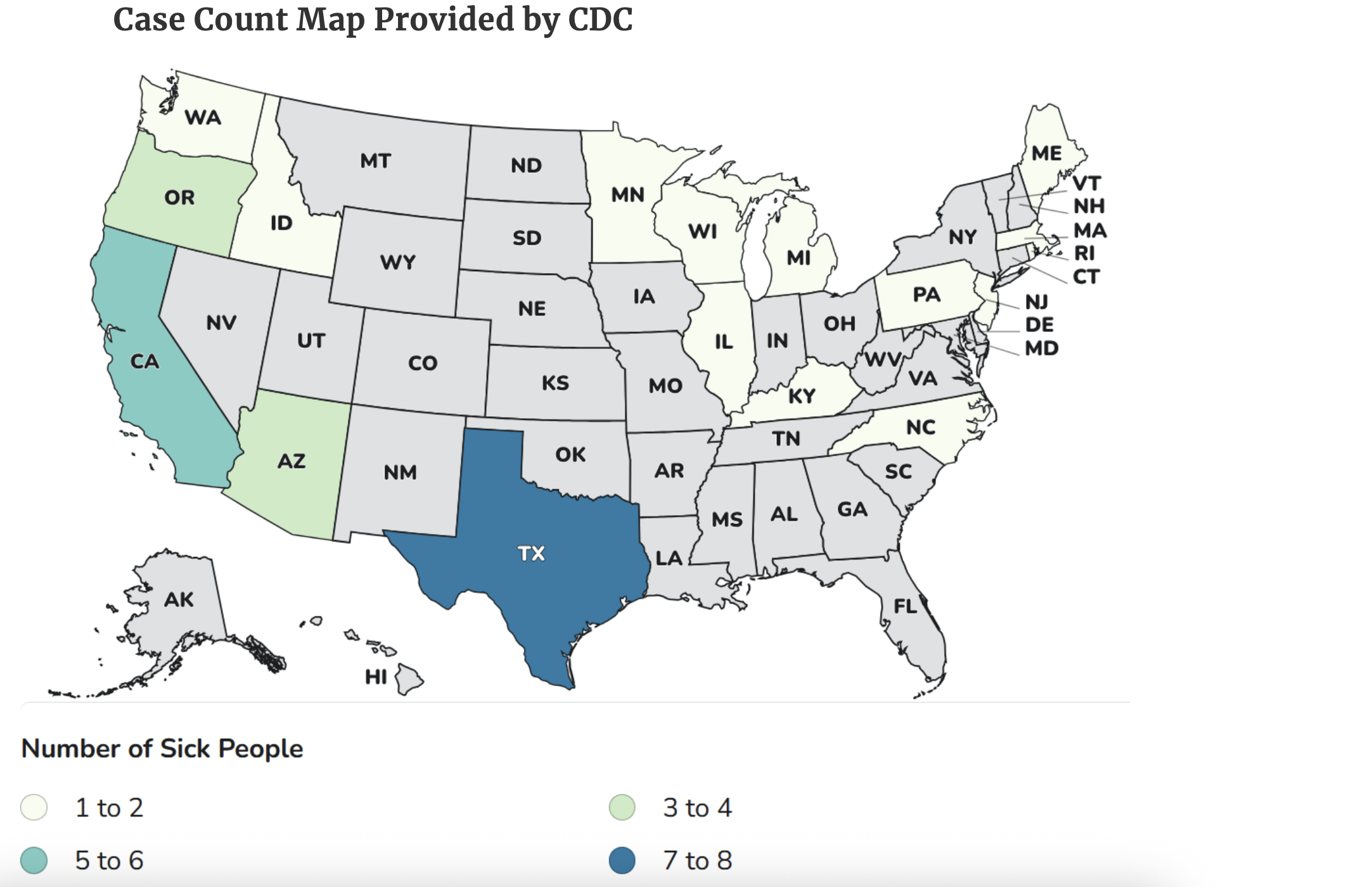
Instances have been documented in Arizona, California, Idaho, Illinois, Kentucky, Maine, Massachusetts, Michigan, Minnesota, North Carolina, New Jersey, Oregon, Pennsylvania, Rhode Island, Texas, Washington, and Wisconsin.
What is infant botulism?
According to Centers for Disease Control and Prevention (CDC), infant botulism is a serious illness affecting the intestines, known medically as intestinal toxemia. The condition begins when an infant swallows spores of the bacterium Clostridium botulinum.
Once swallowed, these spores temporarily colonize the infant’s large intestine and begin producing a potent neurotoxin.
How it affects the body
The danger of botulism lies in how this toxin attacks the nervous system.
The neurotoxin binds to specific nerve endings (cholinergic nerve terminals) and destroys the proteins required to release acetylcholine, the chemical messenger that tells muscles to contract.
When this communication is cut off, the result is a dangerous chain reaction in the infant’s body:
- Bulbar Palsies: Paralysis affecting the face and throat muscles.
- Hypotonia: A distinct loss of muscle tone, often making the baby appear “floppy.”
- Descending Paralysis: A symmetric weakness that typically starts in the face and moves downward through the body.
Signs and symptoms for parents, caregivers should be on high alert for the following symptoms, which can vary in severity:
- Constipation: Often one of the earliest signs.
- Facial Changes: Drooping eyelids (ptosis), sluggish pupil reaction, and a flattened facial expression.
- Feeding Issues: A diminished suck or gag reflex, leading to poor feeding.
- Sound: A weak or altered cry.
- Breathing Trouble: Respiratory difficulty, which can escalate to respiratory arrest in severe cases.
If an infant shows signs of muscle weakness or breathing difficulty, immediate medical attention is required.
Products still found at major retailers
Consequently, FDA also warned that the recalled products are still being found on store shelves across the United States.
State and local public health officials are currently interviewing caregivers regarding feeding habits in the month prior to the infants becoming sick. According to the FDA, all 37 identified cases involved infants fed ByHeart formula.
According to FDA, they received reports that the said formula is still being found on store shelves in multiple states, including at multiple Walmart, Target, and Kroger locations, and at one or more Sprouts Organic Market, Safeway, Acme, Jewel-Osco, Shaw’s, Star Market, Smith’s, King Sooper’s, Albertson’s, Whole Foods, Wegman’s, and Publix locations.
The agency is actively working with state partners to ensure immediate removal, but consumers are urged to be vigilant.
“All ByHeart infant formula products have been recalled, and these products should not be available for sale in stores or online. This includes all formula cans and single-serve ‘anywhere pack’ sticks,” the agency said.
Investigation into manufacturing facilities
The FDA is currently conducting onsite inspections to determine the specific point of contamination. The agency has released inspection reports for three manufacturing facilities owned by ByHeart’s parent company, Blendhouse, located in Iowa, Oregon, and Pennsylvania.
- Allerton, Iowa: Inspected in February 2025, officials cited deficiencies in Good Manufacturing Practices (GMP).
- Reading, Pennsylvania: Inspected in January 2024, this facility received an “Official Action Indicated” classification, the most serious category. This facility has been shuttered since September 2023.
- Portland, Oregon: Inspected in March 2025, this facility was cleared with “No Action Indicated.”
Additional testing by the FDA, CDC, and ByHeart is underway, with results expected in the coming weeks.
International distribution via Amazon
While the primary recall is domestic, online sales have spread the product globally.
Customer data provided by Amazon indicates that limited quantities of the recalled formula were shipped to over 20 international destinations, including Canada, China, Japan, the Philippines, the United Kingdom (British Virgin Islands), and throughout South America.
Health officials also advised consumers to discard any ByHeart brand infant formula immediately.
The Associated Press reported that families whose infants were treated for botulism after consuming ByHeart formula have initiated legal action against the company.
The lawsuits, submitted in federal courts, claim that the formula was faulty and that ByHeart acted negligently in its distribution.
The plaintiffs are pursuing compensation for medical expenses, emotional suffering, and other damages.
Company’s response
Early in November, the company’s founders, Mia Funt and Ron Belldegrun, issued an open letter expressing deep regret, stating,
“We are so sorry for the immense anxiety and fear that we have been causing you these past few days.”
They emphasized that the safety of infants is their highest priority and that they are operating “out of an abundance of caution.”
Furthermore, in an effort to support affected families, ByHeart updated its refund policy. They are now offering a full refund for all products purchased on ByHeart.com on or after August 1, 2025.
Process: They established a dedicated support line (1-866-201-9069) and email ([email protected]) to assist customers with refunds and questions.
Regarding the lawsuits filed by families of sickened infants, the company has generally stated they will “address any legal claims in due course” while maintaining their focus on the recall and family safety.
Also read:
Local breweries warn proposed hemp drink ban could harm jobs
Cincinnati-area charitable pharmacy to receive grant for nutrition program